原名:Low-Protein Diet Supplemented with Medium-Chain Fatty Acid Glycerides Improves the Growth Performance and Intestinal Function in Post-Weaning Piglets
译名:低蛋白日粮添加中链脂肪酸甘油酯可改善断奶仔猪的生长性能和肠道功能
作者:Zhijuan Cui 1,2, Xianze Wang 1 , Zhenping Hou 3 , Simeng Liao 2 , Ming Qi 2 , Andong Zha 2, Zhe Yang 2 , Gang Zuo 1 , Peng Liao 1 , Yuguang Chen 1,* and Bie Tan 1,2,*
完成单位:1College of Animal Science and Technology, Hunan Agricultural University, Changsha 410128, China
2Laboratory of Animal Nutritional Physiology and Metabolic Process, Key Laboratory of Agro-ecological Processes in Subtropical Region, National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, China
3Institute of Bast Fiber Crops, Chinese Academy of Agricultural Sciences, Changsha 410205, China
*Authors to whom correspondence should be addressed
期刊:animals
影响因子:2.4
发表时间:2020-10-12

导读:After weaning, piglets cannot absorb protein well and cannot get enough energy from the diet due to intestinal dysplasia. Medium-chain fatty acids are very effective in providing energy for piglets and may protect the integrity of the intestinal barrier to improve the healthy development of piglets. Therefore, we speculate that medium chain fatty acid glycerides can promote the growth of weaned piglets in a low protein diet. The present study examined the effects of medium-chain fatty acid glycerides on the growth performance, intestinal barrier function and inflammatory response of weaned piglets. These findings provide a new prospect for the application of medium-chain fatty acid triglycerides in piglets.
1、摘要
Medium-chain fatty acid glycerides have been shown to provide energy for rapid oxidation in the body. The study was conducted to investigate the effects of dietary supplementation with medium-chain fatty acid glyceride on the growth performance and intestinal health of weaned piglets fed with a low-protein diet. Nighty healthy weaned piglets were randomly divided into five treatments: NP (Normal protein treatment, normal-protein diet no antibiotics included); NC (Negative control, low-protein diet no antibiotics included); PC (Positive control, low-protein diet +75 mg/kg quinocetone, 20 mg/kg virginiamycin and 50 mg/kg aureomycin); MCT (tricaprylin + tricaprin treatment, low-protein diet + tricaprylin + tricaprin); GML (glycerol monolaurate treatment, low-protein diet + glycerol monolaurate). The results showed that the average daily feed intake (ADFI) of the MCT treatment was significantly higher than that of the NP, NC treatments (p < 0.05). In the jejunum, the villus height of the GML treatment was significantly lower than that of the PC treatment (p < 0.05), and the number of goblet cells in the GML treatment was higher than that in the NC treatment (p < 0.05). Compared with the NC treatment, the MCT treatment significantly increased the level of claudin-1, Zonula occludens-1(ZO-1), while the GML treatment significantly increased the level of claudin-1, occludin, ZO-1 (p < 0.05). In the ileum, the level of ZO-1 in the GML treatment was significantly higher than that in the NP, NC, PC treatments (p < 0.05). Compared with the NC treatment, the GML treatment significantly increased the level of Secretory immunoglobulin A (SIgA) in the ileum and serum, while the MCT treatment significantly increased the level of SIgA and decreased the level of interleukin-6 (IL-6) in the ileum (p < 0.05). These results showed that the addition of medium-chain fatty acid glycerides to a low-protein diet could improve the growth performance and intestinal functional barrier of weaned piglets and also improve the immune function of weaned piglets.
2、试验设计
A total of 90 healthy Duroc×Landrace×Large Yorkshire piglets weaned at 21 days of age (body weight 6±0.15 kg) were randomly assigned to five treatments, with six pens per treatment and three piglets per pen. The study design is shown in Table 1: NP (Normal protein treatment, normal-protein diet no antibiotics included); NC (Negative control, low-protein diet no antibiotics included); PC (Positive control, low-protein diet +75 mg/kg quinocetone, 20 mg/kg virginiamycin and 50 mg/kg aureomycin); MCT (low-protein diet + tricaprylin/tricaprin); GML (low-protein diet +glycerol monolaurate). The normal protein basal diet and low-protein basal diet were formulated according to the nutrient requirements for weanling piglets (NRC, 2012) and previous studies (Table 2). The medium-chain fatty acid glycerides were obtained from Deyuanshun Biological Technology Co., Ltd. (Beijing, China). The experiment lasted for 14 days, and all piglets were freely fed.
On the second morning after the 14th day, we collected the blood and tissue. After overnight fasting, we collected blood from the jugular vein of piglets in the morning. Approximately 10 mL of blood from the jugular vein was collected in aseptic capped tubes containing 150 U of sodium heparin and an ordinary centrifuge tube. Serum and plasma samples were obtained by centrifugation at 3000× g for 10 min at 4 ℃ and were stored at −80 ℃ for biochemical detection. Six piglets from each treatment were anesthetized with sodium pentobarbital (20 mg/kg BW) and killed by jugular puncture. After slaughtering, the samples of jejunum and ileum were immediately snap-frozen in liquid nitrogen and then transferred to −80 ℃ for further analysis. The jejunum and ileum (around 2 cm) was fixed in 4% formalin to detect the morphology of the intestine. The chyme of the colon was collected in a 50 mL centrifuge tube and then transferred to −80 ℃ for a fatty acid determination.
3、结果与分析
表1:Study design.
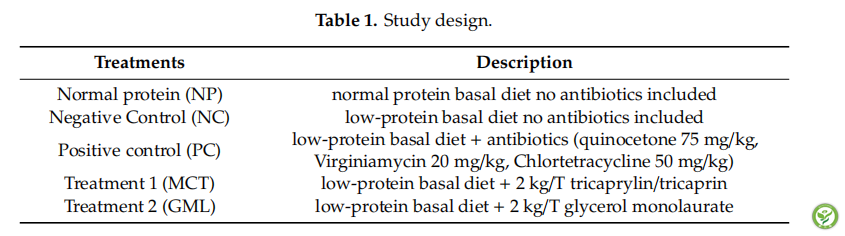
表2:Composition and nutrient levels of the basal diet (as-fed basis).
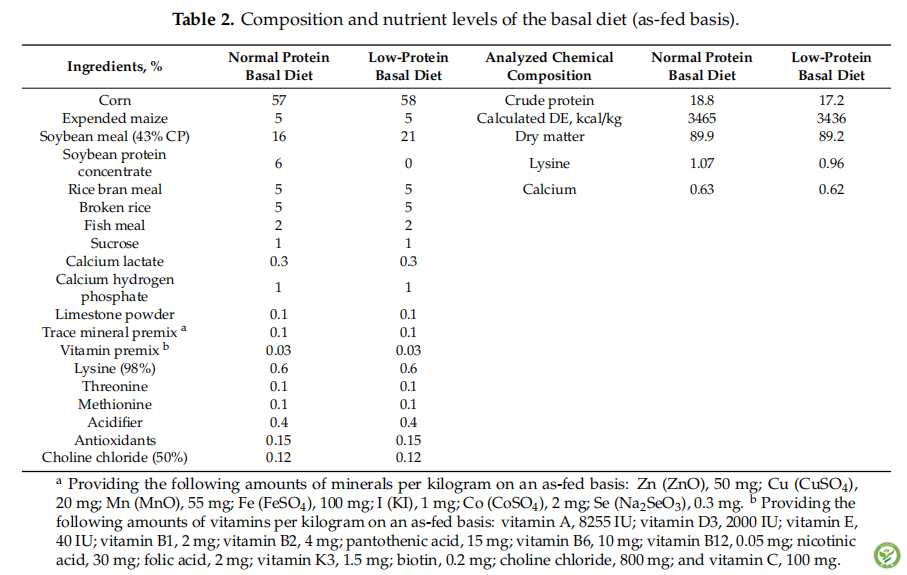
表3:The growth performance of piglets.
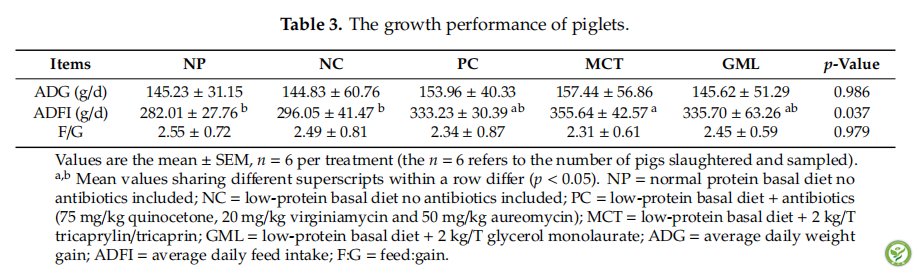
The average daily gain (ADG), average daily food intake (ADFI) and feed/gain ratio of piglets (F/G) are shown in Table 3. There were no differences in ADG and F/G among all treatments (p > 0.05). The MCT treatment significantly increased ADFI when compared with the piglets of the NC and NP treatments (p < 0.05), but there were no differences in ADFI among the PC, MCT or GML treatments (p > 0.05).
表4:The intestinal permeability of piglets.
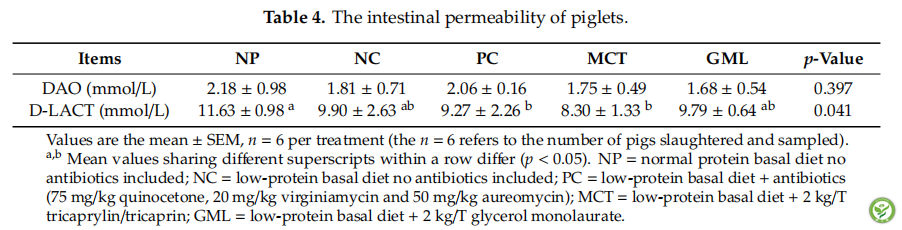
Table 4 shows the indications of the intestinal permeability, serum concentrations of D-LACT and DAO, of piglets. The concentration of D-LACT in the serum of piglets in the MCT treatment was lower than for the NP treatment (p < 0.05), but the MCT and GML treatments did not affect the D-LACT concentration when compared with the NC and PC treatments (p > 0.05).
图1:The morphology and structure of the jejunum in the piglets. (A): The jejunal villus height. (B): The jejunal crypt depth. (C): The jejunal lymphocyte numbers. (D): The jejunal goblet cell numbers. Values are the mean ± SEM, n = 6 per treatment (the n = 6 refers to the number of pigs slaughtered and sampled). a,b Mean values sharing different superscripts within a row differ (p < 0.05). NP = normal protein basal diet no antibiotics included; NC = low-protein basal diet no antibiotics included; PC =low-protein basal diet+antibiotics (75mg/kg quinocetone,20mg/kg virginiamycin and 50mg/kg aureomycin);MCT = low-protein basal diet + 2 kg/T tricaprylin/tricaprin; GML = low-protein basal diet + 2 kg/T glycerol monolaurate.
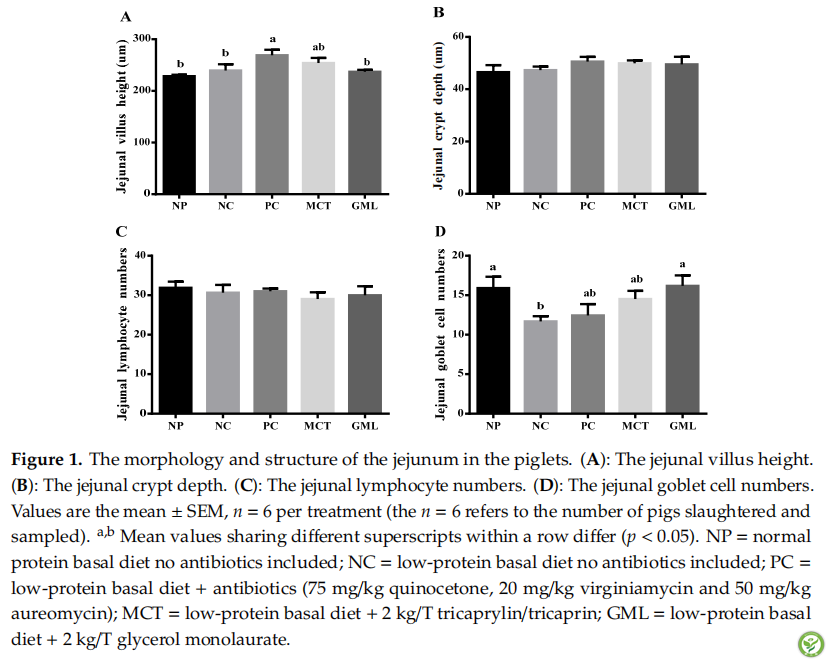
图2:The morphology and structure of the ileum in piglets. (A): The jejunal villus height. (B): The jejunal crypt depth. (C): The jejunal lymphocyte numbers. (D): The jejunal goblet cell numbers. Values are the mean ± SEM, n = 6 per treatment (the n = 6 refers to the number of pigs slaughtered and sampled). NP = normal protein basal diet no antibiotics included; NC = low-protein basal diet no antibiotics included; PC = low-protein basal diet + antibiotics (75 mg/kg quinocetone, 20 mg/kg virginiamycin and 50 mg/kg aureomycin); MCT = low-protein basal diet+2 kg/T tricaprylin/tricaprin; GML = low-protein basal diet + 2 kg/T glycerol monolaurate.
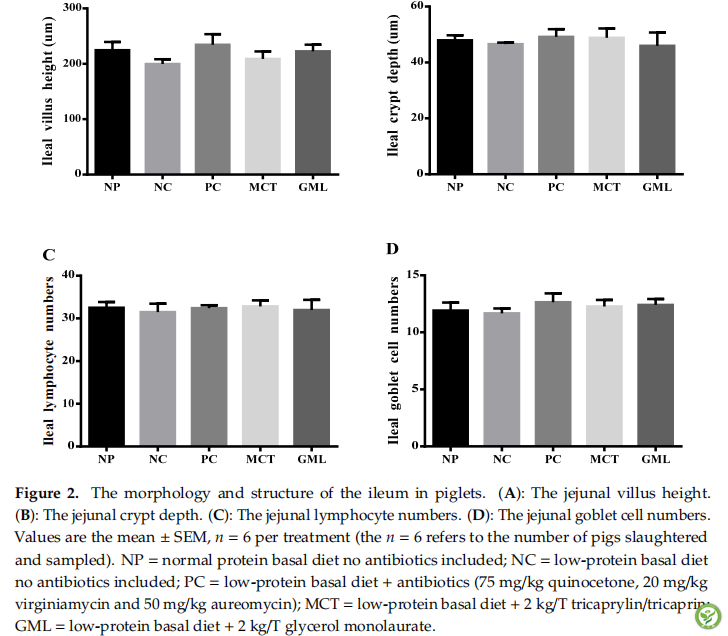
图3:The expressions of tight junction proteins of the jejunum in piglets. (A): The protein expression of claudin-1. (B): The protein expression of occludin. (C): The protein expression of ZO-1. (D): The protein expression of claudin-1, occludin, ZO-1. The (A–C) expressions of tight junction protein and (D) representative immunohistochemical staining images in the jejunum of piglets. Values are the mean ± SEM, n = 4 per treatment (the n = 4 refers to the number of pigs slaughtered and sampled). a,b,c,d Mean values sharing different superscripts within a row differ (p < 0.05). NP = normal protein basal diet no antibiotics included; NC = low-protein basal diet no antibiotics included; PC = low-protein basal diet + antibiotics (75 mg/kg quinocetone, 20 mg/kg virginiamycin and 50 mg/kg aureomycin); MCT = low-protein basal diet+2 kg/T tricaprylin/tricaprin; GML = low-protein basal diet + 2 kg/T glycerol monolaurate.
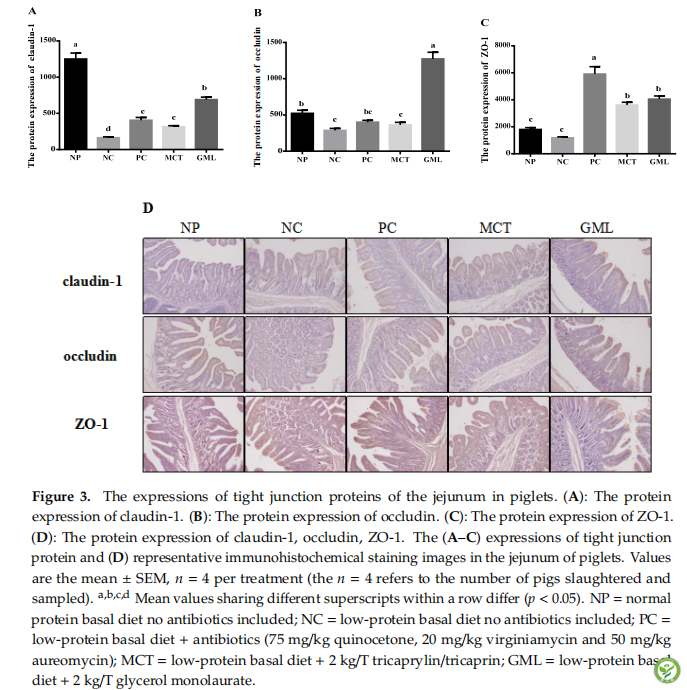
图4:The expressions of tight junction proteins of the ileum in piglets. (A): The protein expression of claudin-1. (B): The protein expression of occludin. (C): The protein expression of ZO-1. (D): The protein expression of claudin-1, occludin, ZO-1. The (A–C) expressions of tight junction protein and (D) representative immunohistochemical staining images in the ileum of piglets. Values are the mean ± SEM, n = 4 per treatment (the n = 4 refers to the number of pigs slaughtered and sampled). a,b,c,d Mean values sharing different superscripts within a row differ (p < 0.05). NP = normal protein basal diet no antibiotics included; NC = low-protein basal diet no antibiotics included; PC = low-protein basal diet + antibiotics (75 mg/kg quinocetone, 20 mg/kg virginiamycin and 50 mg/kg aureomycin); MCT = low-protein basal diet + 2 kg/T tricaprylin/tricaprin; GML = low-protein basal diet + 2 kg/T glycerol monolaurate.
表5:The concentrations of cytokines in the jejunal mucosa of piglets.
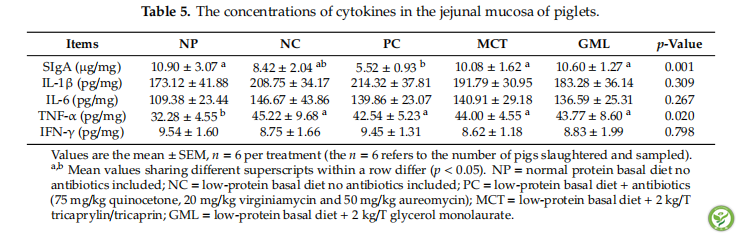
In the jejunal mucosa, there was no significant difference in IL-1β and IL-6, IFN-γ among the treatments. The MCT and GML treatments increased the concentrations of SIgA in piglets when compared with the PC treatment and decreased the concentrations of TNF-α in piglets when compared with the NP treatment (p < 0.05) (Table 5).
表6:The concentrations of cytokines in the ileal mucosa of piglets.
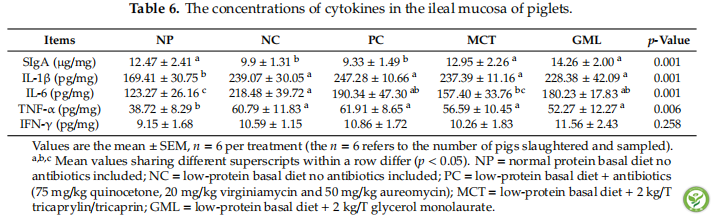
In the ileal mucosa, when compared with the NC treatment, the piglets in the MCT and GML treatments showed an increase in the SIgA concentration, but a decrease in the concentrations of IL-6 and TNF-α (p < 0.05). Additionally, the dietary supplementation with MCT and GML increased (p < 0.05) the SIgA concentration but did not affect (p > 0.05) the concentrations of inflammatory cytokines (IL-1 β, IL-6, TNF-α, IFN-γ) when compared with the PC treatment (Table 6).
表7:The serum concentrations of cytokines in the piglets.
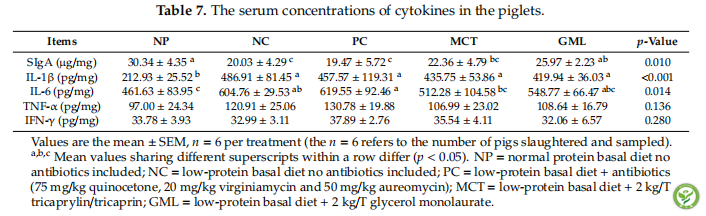
In the serum, the SIgA concentration in piglets of the GML treatment was significantly higher than that of the NC and PC treatments (p < 0.05). MCT addition decreased the IL-6 concentration when compared with the PC treatment, but increased its concentration when compared with the NP treatment (p < 0.05) (Table 7).
4、结论
This study shows that the supplementation of medium-chain fatty acid glycerides to a low-protein diet can decrease the intestinal permeability, improve the intestinal morphology and structure, increase some tight junction protein expressions, promote the secretion of IgA, and inhibit the production of inflammatory cytokines in weaning piglets. These findings illustrate that medium-chain glycerides can improve the growth performance of piglets by improving the intestinal barrier and by regulating cytokines.











